|
THE AGES OF GAIA: A BIOGRAPHY OF OUR LIVING EARTH |
|||||||||||||||||||||||||||||||||||
|
6: Modern Times
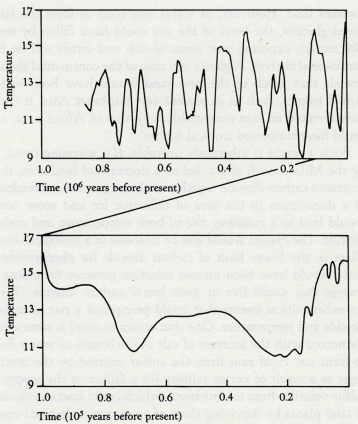
This chapter is about the period of the Earth's history when living organisms large enough to be seen with unassisted eyes were growing or moving on the land and in the sea. The microorganisms were still there flourishing and still responsible for much of the regulation of the Earth. But the arrival of large, soft, bodied cell communities changed the surface of the Earth and the tempo of life upon it: Plants that could stand erect supported by structures of deep underground roots. Consumers that could travel on the ground and in the air or sea. All these things left fossil remains. Their presence delineates this period called the Phanerozoic, going from the Cambrian some 600 million years ago until the present day. Because we live in it, and because recent historical records are so much more detailed than those of the ancient past, it seems a period well known and familiar. This is an illusion. We know little about the Earth even in our own time. For the Cambrian there are just catalogs of species and rocks. They give some insight into the life of the Earth, but only in the abbreviated way that a telephone book does about the private lives and the economy of a town. If we take Gaia to be a living organism, the Phanerozoic can be viewed as the most recent stage in her life, and the one she is still in. This may be easier than considering independently the lives of the billions of organisms from which she is made. Getting to know a friend does not usually require a detailed knowledge of her cellular structure. Similarly, geophysiology, concerned with the whole Earth, need not be too confused by the mass of undecomposed detail that lies, like thick layers of fallen leaves, beneath the branches of the tree of science. So let us look at the physiology of Gaia during this period. In an ideal history the description would be of the whole system, but the habit of reduction dies hard. At the present stage of ignorance it is much easier to divide the chapter into parts, each concerned mainly with the regulation of one important chemical element and of the climate. Geologists see the transition from the Proterozoic to the Phanerozoic as occurring about 570 million years ago. The first organisms that we would recognize as animals with skeletons appeared on Earth somewhat earlier than this. As a geophysiologist I prefer to see this transition as also marked by a change in oxygen abundance, an event not unlike the one that occurred between the Archean and the Proterozoic. My colleagues have made it very clear to me that what follows about oxygen is speculative and often contrary to conventional wisdom. I have included it in spite of their protest because it illustrates a view of the evolution of free oxygen in the light of Gaia theory. Whether it is right or wrong seems to me less important than its value in stimulating new experiments and measurement. So let us consider oxygen. This gas comes from the use of sunlight by the green chloroplasts within cells to convert carbon dioxide and water into free oxygen and the biochemicals from which they are made. Most of the oxygen is used up again by the consumers who eat the plants and algae, oxidize the food, and return carbon dioxide to the air and the sea. From the beginning the producers, the photosynthesizers, have had a love-hate relationship with the consumers. Producers do not care to be eaten, but the presence of the consumers is essential for their health and that of the larger organism they constitute. When plants and animals appeared, the fine details of this constructive aggression became visible. The plants were seen to possess poisons, spines, and stings; and the animals and microorganisms were obliged to develop new techniques for grazing. A balance is always struck because, without the consumers, the survival of the plants and algae would be threatened. There is only a few years' supply of carbon dioxide in the air. The removal of consumers from the scene would be disastrous for plants, and within a short time span. Not only would there be too little carbon dioxide for photosynthesis, but there would be major climate changes as the gases of the atmosphere and the albedo of the Earth responded to the demise of the plants. Not least, the intricate recycling of nutrients and gardening of the soil would cease. On a human time scale the coexistence of consumers and producers could be compared with the long peace that has reigned between the hostile yet mutually dependent superpowers. Oxygen is also used up in its reaction with, for example, the sulfur gases emitted by volcanoes, or the reducing chemicals in the igneous rocks that solidify from the magma emerging from below the sea floor. Oxygen is kept at a constant level by the burial of a small proportion of the photosynthetic carbon, about 0.1 percent, just enough to equal the losses. We know that the level of oxygen must have changed at the end of the Proterozoic, because of the new forms of life that appeared. When the organisms were mostly living in water, or as colonies of algal mats on the surface of the land, the upper limit of oxygen would have been set by its toxicity. For such ecosystems, fires are less a problem than they are to standing vegetation. They could have tolerated an atmosphere containing as much as 40 percent oxygen, provided that the extra atmospheric pressure did not so exacerbate the gaseous greenhouse as to lead to an intolerably hot climate. However, the free-swimming eukaryotes that appeared in the early Proterozoic would not have required much oxygen since the gas could diffuse easily across the small distance between the walls of their microscopic cells; as little as 0.1 percent in the atmosphere may have been sufficient. The larger organisms that appeared in the Phanerozoic, such as the dinosaurs, which were composed of massive volumes of cells in juxtaposition, could have existed only in a richer oxygen environment. This is especially true where there was a need for a greater power output during swimming. Even today, with oxygen at 21 percent, our muscles cannot be supplied with sufficient oxygen at maximum power output; a backup temporary power supply, called glycolysis, operates when we run as fast as we can. Peter Hochachka, in an unusual book called Living Without Oxygen, describes the intricate mechanisms by which large animals cope with the problem of power production in a world which, for them, can be limited in its oxygen supply. An example of this size effect is illustrated by the poison carbon monoxide. For animals as large as ourselves, carbon monoxide is inescapably deadly. It kills by preventing the red blood cells from conveying oxygen to our tissues. A smaller animal, the mouse, can survive the complete saturation of its blood with carbon monoxide. It survives the poison because enough oxygen can diffuse to its tissues from the skin and from the surface of the lungs. There has to be an upper limit of oxygen concentration at which these large animals can live because of the toxic effects of this gas. We are so accustomed to think of oxygen as lifesaving and essential that we ignore its potent toxicity. Oxidative metabolism, the extraction of energy from food through its reaction with oxygen, is inevitably accompanied by the escape of highly poisonous intermediates within the cell. A substance like the hydroxyl radical is such a powerful oxidant that were it present as a gas at the same concentration as oxygen, almost anything flammable would instantly burst into flame. It reacts with methane at room temperature, whereas free oxygen does not until nearly 600°C. Other undesirable products from oxygen are hydrogen peroxide, the superoxide ion, and oxygen atoms. Living cells have developed mechanisms to detoxify all those products: Enzymes, such as catalase, that decompose hydrogen peroxide to oxygen and water, and the superoxide dismutase, which converts the malign superoxide ion to harmless products. Antioxidants, such as tocopherol, that mop up hydroxyl radicals. We and other animals alive today, from the largest to the smallest, owe our life spans to this system of chemical protection developed by our distant bacterial ancestors. If there is no great excess of oxygen, its toxicity can be contained. Why did the level of oxygen rise? At the end of the Archean, the supply of reductants -- sulfides and ferrous iron -- of the early Earth became insufficient to match the flux of oxygen coming from the burial of carbon, and the oxygen increased. It reached a low steady state in the early Proterozoic, much less than in the present atmosphere, representing a balance between the needs of early consumers and the toxicity of oxygen to the early photosynthesizers. There is no similar clear-cut event in the Proterozoic corresponding to the appearance of oxygen at the end of the Archean (see table 6.1). We do not know why the level of oxygen began to rise again, although Robert Garrels proposes that it was associated with the development of bacteria that reduce sulfates. This would have led to the burial of more of the products from the photosynthesizers, as sulfur or sulfides, so leaving behind an excess of oxygen in the air. However it happened, the reactions of this free oxygen with other elements such as carbon and sulfur would release acids into the air, and these would increase the weathering of crustal rocks so that more nutrients were released, leading to a greater abundance of living organisms. The positive feedback on the growth of oxygen would continue until the disadvantages of its presence overcame the benefits. Rather like the growth of car population in some cities, it continues until movement is choked by its presence.
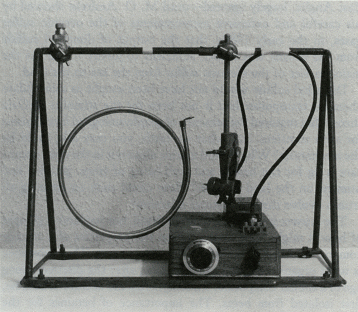
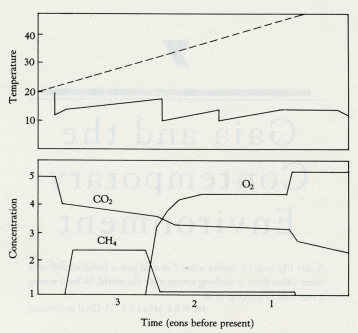
At some time in this period organisms began synthesizing, on a large scale, the precursors of those enigmatic substances, lignins and humic acids. It may have been the result of the invention of some new antioxidants. The precursors of lignins are phenols, well known to react vigorously with hydroxyl radicals. A typical member of this class of acid substances is coniferyl alcohol: when it reacts with hydroxyl it produces lignin, a carbon-containing polymer that has great chemical stability and a resistance to biodegradation. Because of these properties lignin would, if made in quantity, increase the rate of carbon burial, and thus the rate of oxygen production. In a geophysiological fashion, lignin has turned out to be a structural material as important for land plants as the bioceramics of bone and shells are for animals. Just as calcite deposition in cells may have originally been part of a device to lower the concentration of the toxic calcium in the cell fluids, so lignin production may have initially come from a method of detoxifying oxygen. Both of these materials enabled the construction of vast cell communities of a new kind. At first in the oceans, but now in the living organisms we recognize as plants and animals. The model of the evolution of oxygen and carbon dioxide regulation, illustrated in figure 5.4, can be extended to the present day. But it is unable, as it stands, to account for the precise regulation of oxygen observed for the past several hundred million years. Oxygen has been constant at 21 percent by volume in the Phanerozoic. The evidence of this constant high concentration is the presence in the sediments of layers containing charcoal. These can be found as far back as 200 million years. The presence of charcoal implies fires, probably forest fires. This sets sharp limits on the atmospheric oxygen abundance. My colleague, Andrew Watson, showed that fires cannot be started, even in dry twigs, when oxygen is below 15 percent; above 25 percent oxygen, fires are so fierce that even the damp wood of a tropical rain forest would burn in an awesome conflagration. Below 15 percent there could be no charcoal; above 25 percent no forests. Oxygen is 21 percent, close to the mean between these limits. It might be that fires themselves are the regulator of oxygen. There is no shortage of lightning strikes for their ignition. If fires are the regulator it cannot be a simple relationship. Oxygen in the air comes from the burial of carbon. Consumers are efficient, and only about 2 percent of carbon photosynthesized reaches the sediments, where a further 95 percent is returned to the oxidized environment as methane. So only one part in a thousand of the carbon fixed by the plants is buried deep. Combustion, on the other hand, is inefficient. As any charcoal maker will tell you, up to 70 percent of the carbon of wood can remain from a controlled combustion. Fires, therefore, would lead to the burial of much more carbon, because charcoal is entirely resistant to biological degradation. Paradoxically, then, fires lead to more oxygen in the long run. If this grim scenario is followed to a conclusion there would at first be a positive feedback on oxygen, but soon the forests would be so devastated that carbon production would fall to the point where oxygen was near or below its present level. The cycle would then repeat. It is true that the layers of charcoal present in the sediments suggest recurrent fires, but the proportion of buried carbon existing as charcoal is much too small to account for such a cycle. A more subtle regulation involving fire would come from the use of fires by certain species of tree as a weapon to sustain its possession of territory. The conifers and eucalyptus trees have both independently evolved to produce on the forest floor a highly flammable detritus: piles of kindling rich with resin and terpenes that ignite and burn fiercely at a lightning stroke. This contrived form of fire does not damage the tall trees themselves, but is death to competing species such as oaks. Furthermore, these fires leave little charcoal; combustion is nearly complete. So developed is the fire ecology of forests that some conifer species require the heat of fire to release their seeds from the seed capsules. The regulation of oxygen so precisely at the convenient level of 21 percent does suggest that the large plants, flammable and nonflammable, who are the victims and beneficiaries both, play a key part. I can't help wondering if those flammable trees that use fire ecology also carry less lignin than other vegetation. If so, they would be a lesser source of buried carbon and so serve to regulate oxygen at a level where fires did take place but not so fiercely as to do more harm than good. The separate discussion of oxygen is justified by its historical significance; it is almost as if oxygen were the conductor who led the players in their evolutionary orchestra. But we need remember that in Gaia the evolution of the organisms and their environment constitute a single and inseparable process. In addition the cycles of all the elements that make up Gaia are closely coupled among themselves, as well as with the species of the organisms. Attempts to describe the role of each of these parts of the system separately are crippling to insight but made necessary by the unavoidable use of the linear form of written expression. With this thought in mind, and remembering that the geophysiology of oxygen and carbon cannot be separated, let us now look at carbon dioxide. In modern times, carbon dioxide is a mere trace gas in the atmosphere compared with its dominance on the other terrestrial planets or with the abundant gases of Earth, oxygen and nitrogen. Carbon dioxide is at a bare 340 parts per million by volume now. The early Earth when life began is likely to have had 1,000 times as much carbon dioxide. Venus now has 300,000 times as much; and even Mars, with much of its carbon dioxide frozen in the surface, has 20 times as much. James Walker and his colleagues tried to explain the low carbon dioxide of the Earth by a simple geochemical argument. Their model was based on the facts that the only source of the gas is volcanic emission and the only sink its reaction with calcium silicate rock. In their world, life played no part in the regulation of carbon dioxide. As the Sun warmed, two processes took place. The first was an increase in the rate of evaporation of water from the sea and, hence, rainfall; the second, an increase in the rate of the reaction of carbon dioxide with the rocks. Together, these processes would increase the rate of weathering of the rocks and so decrease the carbon dioxide. The net effect would be a negative feedback on the temperature rise as the solar output increased. Unfortunately, this imaginative and plausible model could not explain the facts. The carbon dioxide it predicted for the present was about 100 times more than it is observed to be. James Walker's model can be brought to life by including within it living organisms. If the soil of a well-vegetated region almost anywhere on Earth is examined, the carbon dioxide content is between 10 and 40 times higher than the atmosphere. What is happening is that living organisms act like a giant pump. They continuously remove carbon dioxide from the air and conduct it deep into the soil where it can react with the rock particles and be removed. Consider a tree. In its lifetime it deposits tons of carbon gathered from the air into its roots, some carbon dioxide escapes by root respiration during its lifetime, and when the tree dies the carbon of the roots is oxidized by consumers, releasing carbon dioxide deep in the soil. In one way or another living organisms on the land are engaged in the business of pumping carbon dioxide from the air into the ground. There it comes into contact with, and reacts with, the calcium silicate of the rocks to form calcium carbonate and silicic acid. These move with the ground water until it enters the streams and rivers, on their way to the sea. In the sea, the marine organisms continue the burial process by sequestering silicic acid and calcium bicarbonate to form their shells. In the continuous rain of microscopic sea shells, the products of rock weathering -- sedimented limestone and silica -- are buried on the sea floor and eventually subducted by the movements of plate tectonics. Were life not present, the carbon dioxide from the atmosphere would have to reach the calcium silicate of the rocks by slow inorganic processes like diffusion. To sustain the same soil carbon dioxide as now the atmospheric concentration would have to be even higher, perhaps as much as 3 percent. This is why the Walker model will not work. Considered in this way, we have an explanation for the low carbon dioxide of today's Earth. This great geophysiological mechanism has served since life began as one part of climate regulation. But as the Sun grows hotter, it can have little chance of continuing to keep our planet cool. There is an inverse relationship between the abundance of carbon dioxide and the abundance of vegetation. Assuming that the health of Gaia is measured by the abundance of life, then periods of health will be at times of low carbon dioxide. During the normal healthy state of Gaia, with the comfortable coolness of a glaciation, carbon dioxide is a bare 180 parts per million by volume -- uncomfortably close to the lower limit for the growth of plants. Not surprising is the emergence in the Miocene, some 10 million years ago, of a new type of green plant able to grow at lower carbon dioxide concentrations. These plants have a different biochemistry and are called C4 plants to distinguish them from the mainstream C3 plants. The names C3 and C4 come from a difference in the metabolism of carbon compounds in these two types of plant: the C4 plants are able to photosynthesize at much lower carbon dioxide levels than the older C3 plants. The new C4 plants include some, but not all grasses, whereas trees and broad, leaved plants generally use the C3 cycle. Eventually, and probably suddenly, these new plants will take over and run an even lower carbon dioxide atmosphere to compensate for the increasing solar heat. But it will serve only temporarily, because in as short a time as 100 million years, assuming nothing else had changed, the Sun will have warmed up enough to require a zero carbon dioxide atmosphere to keep the present temperature. As we shall shortly see, there are other cooling mechanisms that could come into play. Also a different ecosystem could evolve that was comfortable with a global mean temperature even as high as 40°C. The carbon dioxide crisis is serious but not necessarily life-threatening to Gaia. If I am right that the glacial cool is the preferred state of Gaia, then the interglacials like the present one represent some temporary failure of regulation, a fevered state of the planet for the present ecosystem. How do they come about? Active systems of regulation or control are well known to exhibit instability when close to the limit of their operating range. This can be clearly seen in the Daisyworld model in figure 3.6 where, as the star warming the imaginary planet grows hotter, the effects of a cyclical plague affecting the plants appear in an amplified form as cyclical fluctuations of temperature until the system fails from overheating. We do not yet know the cause of the glaciations, but we do know that they are a periodic phenomenon, synchronized with small variations in the amount of solar radiation reaching the Earth and with long-term variations in the Earth's inclination and orbit. This astrophysical link between glaciation and the Earth's orbit and inclination was proposed by a Yugoslavian, Milutin Milankovich. The magnitude of the change in warmth received from the Sun is not in itself enough to account for the range of temperature between the glacials and interglacials, but it could be the trigger synchronizing the change from one state to another. According to a Japanese physicist, Shigeru Moriyama, the mathematical analysis of the periodicity of the Earth's mean temperature during the past million years is more consistent with an internal oscillation, triggered externally, than with an oscillation that was free running, or simply a response to the changes in radiant energy received from the Sun. Geophysiology suggests that, to regulate the climate in face of increasing heat from the Sun, glacials are the normal state and the interglacials, like now, are the pathological one. Thinking this way, the low carbon dioxide during the glacials can be explained by the presence of a larger or more efficient biota. There must have been more living organisms on Earth; how else could the carbon dioxide have been so low? If more organisms were doing the pumping, where were they? At first thought it might seem that the ice sheets would leave less room for life as it covered much of what is now, or was before humans, forested land. However, as water was used to form the land-based glaciers, the level of the sea could have fallen by some 100 meters, exposing vast areas of rich and fertile soil on the continental shelves. A glance at a map of the continental shelves reveals that much of the new land would have been in the humid tropics, such as in present-day Southeast Asia. It could have covered an area comparable with that of Africa now, and could have supported tropical forests. Such a world is inherently unstable. If a warming trend, as by the Milankovich effect, led to a decrease of land area, then increased carbon dioxide together with the geophysical feedback of a diminution in the area of reflective ice and snow cover would lead to a runaway rise of both temperature and carbon dioxide. The system would also be unstable in a biological sense. Close to the lower limit of carbon dioxide for photosynthesis there would have been intense selection pressure for plants to emerge that could live at even lower carbon dioxide. There are other critical events that could precipitate a rise of carbon dioxide and temperature. One that comes to mind is some effect connected with the increase of salt in the oceans as water froze to form ice. Acid rain from the sulfur emitted by the marine algae as a result of excess salinity (or a failure of the supply of sulfur volatiles from marine biota, which could lead to a decline of land plants by depriving them of an essential element) could be another. A decrease of cloud cover and planetary albedo is yet another. The cycles of the ice ages are known. Figure 6.1 illustrates the time history of temperature during the past million years. Time (105 years before present) 6.1 Temperature history of the recent series of glaciations. (After S. W. Matthews.) We also need to take into account regional processes that may oppose the general tendency for cooling. In the northern temperate regions the great conifer forests are dark in color and easily shake off or shed the white snow that falls on them in winter. The length of the winter season must be considerably reduced by their presence. The late winter sunshine at continental latitudes greater than 50° is not powerful enough to melt fresh snow; the whiteness of it reflects the radiant energy skywards. Dark pine trees, though, absorb the sunlight and warm not just the trees themselves but the region. Once the snow has melted then even the bare ground can absorb sunlight, making it warm enough for seeds to germinate and letting the spring commence. The circularity of explanations of physiological control systems makes it difficult to choose a point of entry. Which came first, the low carbon dioxide and dense cloud cover, or the low temperature? This question, like that about the priority of chickens and eggs, could be pointless. Let us look instead at a recent evolutionary development, the emergence of the C4 plants that are able to grow at lower concentrations of carbon dioxide than the older C3 plants. These C4 plants could be both the result of the glaciations and an encouragement for further glacial periods. Now there is ample carbon dioxide for all plants, so there is not much competition between C3 and C4 plants for habitats, except through the agency of humans who, in agriculture, remove the older C3 plants and replace them by wheat, rice, bamboo, sugar cane, and so on, many of which are C4 plants. During a glaciation, when the carbon dioxide is near the lower limit tolerable for C3 plants, the advantages of the C4 metabolism begins to tip the balance in their favor. The human propensity to interfere was the plot of a doom scenario in my first Gaia book. The central character was an earnest, well-meaning agricultural biologist, Dr. Intensli Eeger. He succeeded, where all other hazards had failed, in eliminating all life by his meddling. He developed, using genetic engineering, a combined nitrogen-phosphorus fixing microorganism. It was intended to improve the yield of rice grown in the humid tropics so that the hunger of the Third World would at last be overcome. Unfortunately, his organism found a free-living unicellular alga much more to its liking than rice plants. So successful was this combination that it conquered the world. It was a Pyrrhic victory, because the bicultural world of the algal-bacterial combination could not, on its own, maintain planetary homeostasis. I have had a certain guilt about ascribing, even to a fictional character, so awful a punishment for meddling, and it seems only fair to give him a second chance. This time he uses his impressive skill to develop a new form of tree starting with wild oats, one that would operate on the C4 cycle and grow vigorously in the humid tropics. It would have a rich sap, a delicious fruit full of vitamins and nutrients, and an ability to grow well in arid areas. Its plantations could reverse the spread of desert. The replacement of much of the humid tropical forests with Avena eegeriansis at first gave the impression that the bad days of environmental degradation were over. Lush plantations were sprouting everywhere, greening the Sahel and bringing back rain to regions that had been desert for thousands of years. Under the shade of the new trees, the complex tropical ecosystems began to return. Soon it was noticed that the carbon dioxide greenhouse problem was abating; the lush growth of the trees had so increased the rate of carbon dioxide uptake by the soil that the sink was now larger than the source. Some scientists, though, were commenting that cloud cover, and, hence, albedo had increased. There was a fierce scientific debate. In line with current thinking, and encouraged by the generous supply of research funds, theorists blamed the increased cloudiness on the activities of the chemical and nuclear industries. Soon the winter snow was lingering in Moscow, Boston, Chicago, Bonn, and Beijing until May; further north it was snowbound year round. Nuclear power stations and the chloro-fluorocarbon industry were closed down. But, faster than the great urban populations of the Northern Hemisphere could grasp, the world would be deep into the next and greatest glaciation. Gaia would breathe free again, cool and comfortable at a total atmospheric carbon dioxide of 100 parts per million. It would not be long, in Gaia's terms, before the oceans receded from the vast areas of continental shelf. Australia and Papua New Guinea would once again be joined by land covered with an ever-extending forest. The lands and cities of the superpowers of yesterday would nearly all be buried under the glaciers. C4 plants would have taken over, with the help of humankind, and liberated Gaia for the start of another long period of homeostasis -- an ice age to last for millions of years, not just hundreds of thousands. This is an unlikely story, but it does serve to illustrate the way that a punctuation can happen as a result of a change in dominant species. We might be the highest form of animal life, but without doubt trees are the highest form of plant life. A fully developed C4 tree might be formidable competition for the forest trees we now know. Dr. Eeger would have redeemed himself and led humans back into a seemly existence within Gaia. In living organisms, the element sulfur is widely used in structures and functions. So next I would like to explain how information gathered in the past decade has enlarged our understanding of the physiological role of sulfur in Gaia. In the summer of 1971 I attended a Gordon Conference held in New Hampton School in the town of the same name in New Hampshire. The title of the conference was "Environmental Science: Air," and the chairman James Lodge, an atmospheric chemist and a friend. It is no small tribute to his powers of organization that this conference could be said to have marked the start of a deep new interest in the atmosphere that has continued to this day. It was there that I first presented experimental measurements of the halocarbons and sulfur gases in the air. I also learnt that the conventional wisdom about the natural cycle of sulfur was that it required large quantities of hydrogen sulfide to be emitted from the oceans to make up for the losses of sulfur, as the sulfate ion, in the run-off of rivers. Without some return of sulfur, the land organisms would soon have been starved of this essential element. I knew from Professor Frederick Challenger's work at the University of Leeds in the 1950s that many marine organisms emitted sulfur as the gaseous compound, dimethyl sulfide. I also knew, as a one-time chemist, that hydrogen sulfide was rapidly oxidized in water containing dissolved oxygen, and that it stunk. It seemed to me that, on both these grounds, it could not be the major carrier of sulfur from the ocean to the land. On the other hand, that elusive smell of the sea is much like that of dilute dimethyl sulfide. Indeed, once you have smelt this gas, pleasant when diluted, it is recognizable ever after as a significant component of the aroma of fresh fish straight from the sea. It is not part of the smell of fresh fresh-water fish. When I returned home to England I thought that it might be a good idea to go by ship from the Northern Hemisphere to the Southern Hemisphere, measuring the sulfur-carrying gases in the air and the sea to try to find out if dimethyl sulfide were indeed the carrier of sulfur in the natural world. I also wanted to take the opportunity to measure the halocarbon gases, such as are used in aerosol sprays, in the hope that these effectively "labeled" the air and would allow us to observe its movement over the oceans. This was to be the last occasion that I applied for research funds through the regular system of writing a proposal and submitting it to a funding agency. What I sought was a small grant, no more than a few hundred pounds, to make some apparatus and take it by ship from the Northern Hemisphere to the Southern, measuring the gases each day the ship sailed. I should have known better. Both proposals were rejected. To the peer reviewers it was pointless to look for dimethyl sulfide, since it was known that the missing sulfur flux was conveyed by hydrogen sulfide. The second proposal, to look for halocarbons, was rejected as frivolous because it was "obvious" that no apparatus existed sensitive enough to measure the few parts per trillion of chlorofluorocarbons I was proposing to seek. I was lucky in being independent. All that I needed for approval to make the voyage was the agreement of my wife Helen, whose housekeeping funds would be somewhat diminished by the cost of the research. She did not share the opinions of my "peers." I made a simple gas chromatograph (shown in figure 6.2) whose total cost could not have been more than a few tens of pounds. Some kindly civil servants of the Natural Environment Research Council, who also disagreed with their panel of academic advisers, provided my travel and subsistence expenses from a discretionary fund. I traveled on a research ship, the RV Shackleton, on its journey from Wales to Antarctica and back. I returned from Montevideo after three weeks on the ship, sadly all the time I could afford; but a fellow voyaging scientist, Roger Wade, kindly continued the measurements when the ship was in Antarctica. My colleague, Robert Maggs, flew out to Montevideo in the spring of 1972 to complete the run home across the equator to Britain. The measurements made on this voyage were reported in three small papers in Nature. The first reported the halocarbon measurements, which showed that the chlorofluorocarbons were persistent and long-lived in the Earth's atmosphere, and that two other halocarbon gases, carbon tetrachloride and methyl iodide, were to be found wherever the ship sailed. These findings led to among other things, the "ozone war" and to the disbursement of an ocean of research funds, recommended by the same committees that had rejected the first applications. Speculations about the threat to "the Earth's fragile shield," the ozone layer, were more plausible than the idea of a voyage of discovery stimulated by no more than the curiosity of an individual scientist. 6.2 Homemade apparatus used to measure gases, in the sea and air, aboard the RV Shackleton on its voyage from Britain to Antarctica and back in 1971 to 1972. The second and third papers on the sulfur gases reported the ubiquitous presence of dimethyl sulfide and carbon disulfide in the oceans. These findings were, apart from the pioneering calculations of the fluxes by Peter Liss of the University of East Anglia, largely ignored -- until M. O. Andreae showed by his careful and extensive measurements of the oceanic sulfur gases, in the early 1980s, that the output of dimethyl sulfide from the oceans was indeed sufficient to justify its role as the major carrier of the element sulfur from the sea to the land. Dimethyl sulfide would not have been sought as a candidate chemical transporter had it not been for the stimulus of Gaia theory that required the presence of geophysiological mechanisms for such transfers. But what on earth, you may ask, could be the mechanism? Why should marine algae out in the open oceans care a fig for the health and well-being of trees, giraffes, and humans on the land surfaces? How could such an amazing altruism evolve through natural selection? The answer is not yet known in detail, but we have a glimpse of how it might have evolved from the properties of a strange compound called dimethylsulfonio propionate. This substance is what organic chemists call a betaine, after the discovery long ago of a similar compound, trimethylammonio acetate or betaine, first isolated from beets. The importance of betaines for the health of marine organisms living in a salty environment was discovered by A. Vairavamurthy and his colleagues. Betaines are electrically neutral salts. They carry a positive charge, associated with the sulfur or nitrogen, and a negative charge, associated with the propionic acid ion, on the same molecule. In an ordinary salt, such as sodium chloride, solution in water separates the charges, which become independent free-floating ions. As we saw in the preceding chapter, marine life lives near the limit of tolerable salt concentration. Salt concentrations above 0.8 molar for sodium chloride are toxic, but this does not apply to betaines. The internal neutralization of their ionic charges renders them nontoxic as salts, and they act in a cell like sugars, glycerol, and the other neutral solutes. Cells that are able to substitute a large proportion of betaine for salt are at an advantage. I wonder if some time, long ago, marine algae were left by the ebbing tide on some ancient beach. The sunlight would soon dry them. As water evaporated from their cells, the internal salt concentration would rise above the lethal limit, and they would die. In the way of evolution, those algae that had present in their cells neutral solutes like the betaines would be less damaged by desiccation and would tend to leave more progeny. In time, the synthesis of betaines would be common among marine algae. Sulfur is plentiful in the sea, whereas nitrogen is often scarce. On the land the reverse is true. This may be why dimethylsulfonio propionate was the chosen betaine rather than the nitrogen betaine of beets and other land plants. (Incidentally beets, also, are able to deal with high concentrations of salt.) This may not be the whole explanation of the presence of dimethylsulfonio propionate as a prominent algal betaine, but there is no doubt that algae that contain it are the source of dimethyl sulfide. When the algae die or are eaten, the sulfur betaine decomposes easily to yield the acrylic acid ion and dimethyl sulfide. Algae that were prone to being left high and dry on the beach would, therefore, have evolved this sulfur gas, and onshore breezes would have carried it inland where atmospheric reactions would slowly decompose it and deposit sulfur as sulfate and methanesulfonate on the ground. Sulfur is scarce on the land and this new source could have enhanced the growth of land plants. The increased growth would increase rock weathering and so increase the flow of nutrients to the ocean. It is not difficult to explain the mutual extension of the land-based ecosystems from the supply of sulfur and of the sea-based ecosystems from the increased flux of nutrients. By this, or some similar series of small steps, the intricate geophysiological regulation systems evolve. They do so without foresight or planning, and without breaking the rules of Darwinian natural selection. Before leaving the beach, so to speak, I have wondered also about the widespread production of methyl iodide by marine plants. Unlike the innocuous dimethyl sulfide, this compound is toxic. It is a mutagen and a carcinogen. The first stimulus for its production may have been as an antibiotic to help the algae to compete, or to discourage predators. The release of methyl iodide to the air from the sea is an essential mechanism for the maintenance of a continuous supply of iodine, an element that is vital for land organisms. It might be worth investigating the possibility that a specific betaine, methyliodonio propionate, exists in large algae such as the brown seaweed, Laminaria, which are a strong source of methyl iodide. If it does, then it suggests a common link with the sulfur betaine story. But there is more to the sulfur and iodine cycles than just the recycling of nutritious elements. The Alaskan geophysicist, Glen Shaw, had a stimulating idea for an efficient geophysiological climate control system. Knowing that a small (in Earthly terms) quantity of sulfur in the stratosphere could profoundly affect the climate, he proposed that the emission of sulfur gases by marine organisms was the most efficient method of climate control. There is a fair body of evidence to suggest that major volcanic eruptions are followed by a global fall in mean surface temperatures. The volcanic gases injected into the stratosphere by the eruption include sulfur dioxide and hydrogen sulfide. (The volcanic cloud also contains an aerosol of solid material, but this soon settles downward.) The sulfur gases remaining in the stratosphere oxidize and, with the water vapors present there, form submicroscopic droplets of sulfuric acid. Because they are so small, they settle only slowly and may persist for several years. These droplets form a white haze in the stratosphere that returns to space the sunlight that might otherwise warm the Earth. Between eruptions, there remains a background of sulfuric acid droplets that are continuously formed from the oxidation of sulfur gases from living organisms. The most important of these are carbonyl sulfide and carbon disulfide. They are minor emissions compared with that of dimethyl sulfide, but in the lower atmosphere they are only slowly oxidized (carbonyl sulfide is especially slow), and persist long enough to enter the stratosphere and be oxidized there. Glen Shaw's proposal was that global overheating could be offset by marine life increasing its output of carbonyl sulfide and carbon disulfide, leading to a thickening of the haze of sulfuric acid droplets in the stratosphere and so to a cooling of the Earth. This may indeed be one of several available geophysiological mechanisms for climate regulation. But it set my colleagues thinking of what might be a much more potent use of sulfur gases for the same end. During extensive investigations of the world oceans, M. O. Andreae has shown that marine organisms emit vast quantities of dimethyl sulfide. These emissions are particularly marked over the "desert" areas of the open oceans far away from the continental shelves. This finding led the meteorologists Robert Charlson and Stephen Warren to propose that the rapid oxidation of dimethyl sulfide in the air over the ocean could be the source of the nuclei needed for the condensation of water vapors to form clouds. Small droplets of sulfuric acid are ideal for this purpose, and over the open oceans there is no other significant source of condensation nuclei from which to form clouds. The aerosol of sea salt which might be thought to nucleate cloud droplets is much less efficient than are the microdroplets of the sulfur acids. The oceans cover about two-thirds of the Earth's surface and their color is a dark blue. Anything that affected the cloud cover over the oceans could powerfully affect the climate of the Earth. In a joint paper, the four of us have reported calculations to estimate the effect that the present natural emissions of dimethyl sulfide could have; these suggest that it is comparable in magnitude with that of the carbon dioxide green-house, but in opposition to it. We have shown the possibility of a powerful link between the growth of algae on the ocean surface and the climate. As a geophysiologist I would further ask: Could these processes serve as a significant part of a responsive climate regulation system? And if so, how did this system evolve? We may also need to take into account the iodine cycle, because the oxidation of dimethyl sulfide in the marine atmosphere is catalyzed by iodine compounds. The production of methyl iodide by the algae may also be a part of this system of climate control. The sites we are proposing for cloud regulation by sulfur emission are the open desert areas of the tropical oceans, about 40 percent of the surface area of the Earth. These regions are low in productivity compared with the continental shelves and inshore waters. They are bare of life, like the great land deserts that span the 30° latitudes north and south of the equator. On land, it is a lack of water that makes the desert; on the oceans it is a lack of nutrients, particularly nitrogen. What are these ocean deserts like? Their waters are clear and blue and, like land deserts, they are by no means devoid of life. One of these deserts is the Sargasso Sea. I recall reading when I was a boy an adventure story about the perils faced on a sailing ship trapped in the dense entangling weed of the Sargasso Sea. When I passed right through that region in 1973 aboard the German research ship Meteor, I was amazed at the difference between the reality and my recollections of the story. There was floating weed, but no more than well-dispersed thin strands of bladder wrack -- the ocean equivalent of sagebrush in an arid desert, and no more impediment to the motion of the ship than would be the sagebrush to walking across the desert floor. The algae at the surface of these ocean deserts do not produce the precursors of cloud condensation nuclei for our benefit nor as a part of some grand design to keep the planet cool. The process must have its origins in the local environmental effects of algal biochemistry. I have discussed the possibility that the production of the sulfur betaine, dimethylsulfonio propionate, may have been a cellular response to salt stress. Although it may have been discovered by marine algae drying out on the shore, successful inventions tend to spread. The concentration of salt in the sea is always uncomfortably high for living organisms. For the unicellular or small-floating organisms, unable to regulate their internal salt by osmotic pressure, synthesizing betaines may have been the cheapest way, in terms of energy, of achieving a low-salt interior. Again dimethylsulfonio propionate would have been the natural choice, because sulfur (in the convenient form of the sulfate ion) is abundant, whereas nitrogen is not. The dimethylsulfonio propionate persists in the cells of the algae during their lifetime, but when they die or are eaten it disperses in the ocean, where it slowly decomposes to yield dimethyl sulfide. Both of these compounds are consumed by other organisms, but there is a steady flux of dimethyl sulfide to the air. In the air, the gas is rapidly oxidi4ed by the ubiquitous hydroxyl radicals until nearly all is converted to sulfuric and methanesulfonic acids. The vapors of these acids are carried aloft by the motions of the air until they reach the heights supersaturated with water vapor, where they act as cloud droplet nuclei. The escape of dimethyl sulfide to the air can bring to the algae inadvertent benefits. The extra cloud cover from the presence of sulfuric acid nuclei changes the local weather. Timothy Jickells of the University of East Anglia has drawn my attention to the fact that clouds over the ocean increase wind velocity, and stir the surface waters, mixing in the nutrient-rich layers beneath the depleted photosynthesizing zone. This is an effective reward for the production of cloud condensation nuclei, and has just been confirmed by the work of the meterologist, John Woods. I doubt if the fresh water of the rain assists much with the salt-stress problem of the algae, but it is no disadvantage. In some regions of the sea, the air carries an aerosol of dust particles blown from the continents; such dust is well known to travel thousands of miles across the oceans. Professor J. M. Prospero, a geophysicist, has regularly found Saharan dust in the air over the West Indies. The Hawaiian islands similarly receive dust from the Asian continent some 4,000 miles distant. The mineral content of this dust when rained out onto the sea may also help the nutrition of the algae there. The surface of the dust particles is not such as to make them suitable as cloud condensation nuclei, but they are washed out of the air by rain induced by the dimethyl sulfide. Lastly, the clouds formed above the ocean filter the radiation reaching the water surface and reduce the proportion of potentially harmful short, wavelength ultraviolet. Visible light needed for photosynthesis is not a limiting factor in the nutrient-poor ecosystems of the oceans, so the shading effect of the clouds is not an adverse one. None of these effects is large, but taken together they may be enough to improve the meadows of the sea and enable the algal species there to leave more progeny. The geophysiological system requires the continuing production of dimethylsulfonio propionate and of the algae that make it. The difficult question is, How does this system become a part of global climate regulation? The oceans become saltier when water freezes out as ice on the polar surfaces; this might lead to increased emission of dimethyl sulfide, increased cloudiness, and so a positive feedback on further cooling. It might be that the greater biomass associated with the glaciations provides more nutrient for ocean life and so sustains the algae. As I write, our first scientific paper on this affair has been published in Nature. These are the early days of this research, and already it looks like becoming an exciting scientific area for research. Two groups of French glaciologists -- Robert Delmas and his colleagues, and C. Saigne and M. Legrand -- have recently reported their discovery of sulfuric and methane sulfonic acids in antarctic ice cores, going from the present to 30,000 years ago. Their data shows a strong inverse correlation between global temperature and the deposition in the ice of these acids. Sulfuric acid has several natural sources, but methane sulfonic acid is unequivocally the atmospheric oxidation product of dimethyl sulfide. There was 2 to 5 times larger a deposition of this sub, stance during the ice age and it seems probable that this was due to a greater output from the ocean ecosystems. If confirmed, it suggests that cloud cover and low carbon dioxide operated in synchrony as part of a geophysiological process to keep the Earth cool. More conservative scientists favor a geophysical explanation arising from the theory of the ocean scientist W. S. Broecker, who has proposed that the glaciations are associated with large-scale changes in the circulation of water in the oceans. Certainly the increase in supply of nutrients that would accompany such an event would alter biological productivity and hence the rate of removal of carbon dioxide and the production of dimethyl sulfide. It looks like becoming an interesting debate. I thought that it might be useful to end this section with a geophysiologist's view of the evolution of the climate and the chemical composition of the atmosphere (see figure 6.3). It is a view of long periods of homeostasis punctuated by large changes. Time (eons before present) 6.3 A geophysiologist's view of the evolution of the climate and atmospheric composition during the life span of Gaia. The upper panel compares the probable temperatures in the absence of life with the stepped but long-term constancy of the actual climate. The lower panel illustrates the stepped fall of carbon dioxide steadily from 10 to 30 percent to its present low level of about 300 parts per million. The early dominance of methane and later of oxygen is also shown. The scale of gaseous abundance is in parts per million by volume and in logarithmic units so that 1 equals 10 parts per million and 5 equals 100,000 parts per million. We seem to be approaching the end of one of these long stable periods. When life began, the Sun was less luminous and the threat was overcooling. In the middle ages of the Proterozoic, the Sun shone just right for life and little regulation was needed, but now it grows hot and overheating becomes an ever-increasing threat to the biosphere of which we are a part.
|
|||||||||||||||||||||||||||||||||||